-- Low-dose povetacicept (80 mg administered once every four weeks) was well tolerated during subcutaneous administration and reduced UPCR by greater than 50% in IgA nephropathy --
-- Higher dose povetacicept (240 mg) administered once every four weeks currently being explored --
-- Based on this data Alpine will now seek to begin a pivotal phase 3 IgAN study in the second half of 2024 --
-- Company to host virtual investor call and webcast today at 4:30 pm ET with James Tumlin, M.D. and Jonathan Barratt, Ph.D., FRCP --
Alpine Immune Sciences, Inc. (NASDAQ: ALPN), a leading clinical-stage immunotherapy company focused on developing innovative treatments for autoimmune and inflammatory diseases, announced that the Company today presented the first clinical data of povetacicept in autoimmune glomerulonephritis during a late-breaking poster session at the American Society of Nephrology (ASN) Kidney Week in Philadelphia, Pennsylvania. In addition, the Company plans to host an investor event today at 4:30 pm ET.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20231102047423/en/
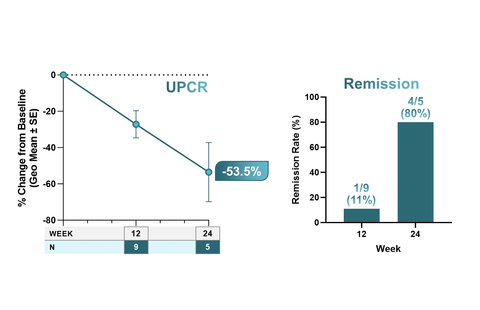
Figure 1. Clinically Meaningful Improvements in Proteinuria, Suggesting Remission (Graphic: Business Wire)
Povetacicept is a potent dual antagonist of the BAFF (B cell activating factor) and APRIL (a proliferation inducing ligand) cytokines, which play key roles in pathogenesis of multiple autoimmune diseases via their roles in the activation, differentiation and/or survival of B cells, particularly antibody-secreting cells, as well as T cells and innate immune cells. RUBY-3 is a multiple ascending dose, multi-cohort, open label, phase 1b/2a study of povetacicept in autoimmune glomerulonephritis, where povetacicept is administered subcutaneously (SC) once every four weeks for up to 48 weeks.
Key Highlights from the Late-Breaking ASN Poster Include:
- As of October 25th, 20 participants with IgA nephropathy (IgAN) have been enrolled, 12 at the 80 mg dose level, of whom 5 have UPCR data available at 24 weeks.
- In IgAN, treatment with low-dose povetacicept, 80 mg SC every four weeks was associated with clinically meaningful improvements in proteinuria, with a 53.5% reduction from baseline in urine protein to creatinine ratio (UPCR; n=5) at 24 weeks. In addition, at 24 weeks, 4/5 (80%) had achieved remission, as defined as UPCR < 0.5 g/g and ≥50% reduction in UPCR from baseline with stable renal function (≤ 25% reduction in eGFR from baseline). (Fig. 1)
- In IgAN, treatment with low-dose povetacicept was also associated with a >60% reduction in the key disease-related biomarker galactose-deficient IgA1 (Gd-IgA1), as well as stable renal function as assessed by estimated glomerular filtration rate (eGFR) (+7.1% from baseline at 24 weeks; n=5).
- The first participant with primary membranous nephropathy (pMN), also treated with povetacicept 80 mg SC every four weeks, achieved an immunological remission, defined as a reduction in the highly disease-relevant biomarker anti-PLA2R1 to an undetectable level, from a baseline of 209 to < 2 RU/mL by 22 weeks.
- Povetacicept has been well tolerated, with no reported administration-associated reactions, no instances of IgG < 3 g/L, and no severe infections.
- A higher dose of povetacicept, 240 mg SC every four weeks, continues to enroll, with initial data expected in 1H 2024.
“In this initial experience, low-dose povetacicept at 80 mg has been well tolerated and demonstrates highly encouraging improvements in UPCR and disease biomarkers in IgA nephropathy, with early evidence suggesting remission," noted James Tumlin, M.D., Professor of Medicine at Emory University School of Medicine, Founder and Chief Executive Officer of NephroNet Clinical Trials Consortium. “These findings suggest a highly compelling development profile for povetacicept based upon a rapid reduction in the key pathogenic biomarker Gd-IgA1, marked and clinically meaningful reductions in UPCR, and a once-a-month dosing regimen. A higher dose of povetacicept 240 mg every four weeks is currently being explored and will be of great interest. Further clinical development of povetacicept in glomerulonephritis, particularly IgAN, is therefore strongly supported.”
Dr. Tumlin continued, “There remains a significant unmet need for well-tolerated, convenient, and efficacious therapies in autoimmune kidney diseases, and dual BAFF/APRIL inhibition has the potential to be an important disease-modifying mechanism, especially if considered early in the treatment paradigm.”
“Even at this low 80 mg dose level of povetacicept, the improvements in proteinuria, accompanied by analogous changes in Gd-IgA1, are impressive and support its potential to be an important therapy for IgAN,” remarked Jonathan Barratt, PhD, FRCP, Mayer Professor of Renal Medicine at the University of Leicester. “Based on this data, advancement of povetacicept to a pivotal trial in IgA nephropathy, where it has already accumulated a reasonably sized clinical experience, seems urgently warranted. The initial findings in primary membranous nephropathy, a disease with no approved therapies, are equally encouraging. Further study is clearly warranted and confirmation of the results in additional pMN patients, along with correlation with renal outcomes, may facilitate a rapid development path.”
“These results are the first of what will hopefully be several data readouts over the next 12-18 months that will help validate the potential activity of povetacicept across multiple autoimmune diseases,” noted Stanford Peng, MD, PhD, President and Head of Research and Development at Alpine. “We eagerly await the initial data from the next 240 mg dose level in the first half of 2024. In the meantime, these data support our plan to advance povetacicept rapidly to a pivotal trial in IgAN, pending regulatory agreement, in the second half of next year. In addition, we plan to initiate RUBY-2, a phase 2 study of povetacicept in systemic lupus erythematosus.”
American Society of Nephrology – Kidney Week 2023
Date/Time: Thursday, November 2, 2023, 10:00am – 12:00pm ET
Poster Title: Povetacicept, an Enhanced Dual BAFF/APRIL Antagonist, in Autoantibody-Associated Glomerulonephritis (GN)
Poster Board Number: TH-PO1125
Session Name: Late-Breaking Posters
Location: Exhibit Hall, Pennsylvania Convention Center, Philadelphia
Presenter: James Tumlin, M.D., Professor of Medicine at Emory University School of Medicine, Founder and Chief Executive Officer of NephroNet Clinical Trials Consortium
Investor Conference Call and Webcast Information
Date/Time: Thursday, November 2, 2023, at 4:30 pm – 5:30 pm ET
Alpine will host a conference call and webcast to discuss the data update from ASN as well as provide a corporate update. Members of the Alpine executive team will be joined by James Tumlin, M.D., Professor of Medicine at Emory University School of Medicine, Founder and Chief Executive Officer of NephroNet Clinical Trials Consortium, and Jonathan Barratt, Ph.D., FRCP, the Mayer Professor of Renal Medicine at the University of Leicester.
The link to the webcast is available in the investor relations section of the Company’s website at https://ir.alpineimmunesciences.com/events and a replay will be available on the Company's website for 90 days following the live event.
About Povetacicept (ALPN-303)
Povetacicept (ALPN-303) is a dual antagonist of the BAFF (B cell activating factor) and APRIL (a proliferation inducing ligand) cytokines, which play key roles in pathogenesis of multiple autoimmune diseases via their roles in the activation, differentiation and/or survival of B cells, particularly antibody-secreting cells, as well as T cells and innate immune cells. Based upon an engineered TACI (transmembrane activator and CAML interactor) domain, povetacicept has exhibited greater potency in preclinical studies versus other inhibitors of BAFF and/or APRIL alone and B cell depletion. Povetacicept is in development for multiple autoimmune diseases, including IgA nephropathy and other autoimmune kidney diseases, systemic lupus erythematosus, and autoimmune cytopenias.
About RUBY-3
RUBY-3 (NCT05732402) is a multiple ascending dose, multi-cohort, open label, phase 1b/2a study of povetacicept in autoimmune glomerulonephritis, where povetacicept is being administered subcutaneously for up to 48 weeks. Key endpoints include proteinuria, eGFR, renal response, and disease-related autoantibodies.
About Alpine Immune Sciences
Alpine Immune Sciences is committed to leading a new wave of immune therapeutics. With world-class research and development capabilities, a highly productive scientific platform, and a proven management team, Alpine is seeking to create first- or best-in-class multifunctional immunotherapies via unique protein engineering technologies to improve patients’ lives. Alpine has entered into strategic collaborations with leading global biopharmaceutical companies and has a diverse pipeline of clinical and preclinical candidates in development. For more information, visit www.alpineimmunesciences.com. Follow @AlpineImmuneSci on X and LinkedIn.
Forward-Looking Statements
This release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. These forward-looking statements are not based on historical fact and include statements regarding our platform technology and potential therapies; the progress and potential of our development programs and product candidates; future development plans and clinical and regulatory milestones and objectives, including the timing and achievement thereof; the efficacy of our clinical trial designs; anticipated enrollment in our clinical trials and the timing thereof; expectations regarding the anticipated reporting of data from our ongoing and planned clinical trials and potential publication of future clinical data; our ability to potentially advance povetacicept directly into a pivotal trial in 2024; and the potential efficacy, safety profile, addressable market, regulatory success and commercial or therapeutic potential of our product candidates. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions and include words such as “may,” “will,” “should,” “would,” “expect,” “plan,” “intend,” and other similar expressions, among others. These forward-looking statements are based on current assumptions that involve risks, uncertainties, and other factors that may cause actual results, events, or developments to be materially different from those expressed or implied by such forward-looking statements. These risks and uncertainties, many of which are beyond our control, include, but are not limited to: clinical trials may not demonstrate safety and efficacy of any of our product candidates; any of our product candidates may fail in development, may not receive required regulatory approvals, or may be delayed to a point where they are not commercially viable; we may not achieve additional milestones in our proprietary or partnered programs; the impact of competition; adverse conditions in the general domestic and global economic markets; we may be unable to advance povetacicept directly into a pivotal trial in 2024; the impact of pandemics, or other related health crises on our business, research and clinical development plans and timelines and results of operations, including the impact on our clinical trial sites, collaborators, and contractors who act for or on our behalf; as well as the other risks identified in our filings with the Securities and Exchange Commission. These forward-looking statements speak only as of the date hereof and we undertake no obligation to update forward-looking statements, and readers are cautioned not to place undue reliance on such forward-looking statements.
Source: Alpine Immune Sciences, Inc.
View source version on businesswire.com: https://www.businesswire.com/news/home/20231102047423/en/
Contacts
Media and Investor Relations Contact:
Temre Johnson
Alpine Immune Sciences, Inc.
ir@alpineimmunesciences.com
media@alpineimmunesciences.com














