Products announced today:
- Two frontotemporal dementia (FTD) disease models provide a scalable and reproducible system to model neurodegenerative disease in a human context.
- ioGABAergic Neurons with unprecedented purity provide a human model for research into neurological diseases including epilepsy, schizophrenia, autism and Alzheimer’s.
Cell coding company bit.bio today announces three additions to its product portfolio: ioGlutamatergic Neurons MAPT N279K and ioGlutamatergic Neurons MAPT P301S disease models, and early access to its ioGABAergic Neurons.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221123005007/en/
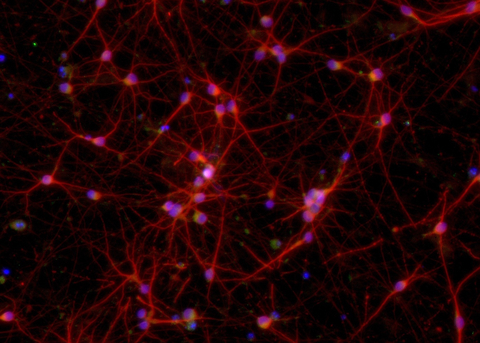
Image of ioGlutamatergic Neurons MAPT P301S demonstrating the cells have a defined glutamatergic neuron identity through the staining of protein markers with fluorescent antibodies (Graphic: Business Wire)
This announcement expands the company’s central nervous system (CNS) portfolio to four disease models and three cell types. bit.bio’s cell products are reprogrammed from human induced pluripotent stem cells (hiPSCs) using the company’s proprietary precision reprogramming technology opti-oxTM.
Dr Mark Kotter, CEO and Founder of bit.bio said:
“At bit.bio our vision is to accelerate biomedical innovation and usher in a new generation of cures through precision reprogrammed human cells. These latest products take us another step closer to that vision and our momentum will continue - we’ve now launched six new products in the past six months with more to come in the new year.”
FTD is the second leading cause of early onset dementia following Alzheimer’s disease. Patients with FTD suffer from behaviour and personality changes and lose their ability to perform daily tasks.
Around a third of FTD cases are hereditary and have been linked to mutations in the MAPT gene. ioGlutamatergic Neurons MAPT N279K and ioGlutamatergic Neurons MAPT P301S each contain different mutations in the MAPT gene commonly observed in FTD patients.
For the first time, scientists can now use these MAPT disease model cells alongside a genetically matched control, ioGlutamatergic Neurons. This means that any experimental differences between the control and the disease model can be confidently attributed to the effects caused by the mutations in the MAPT gene, offering researchers a new window into the disease mechanisms of FTD and in identification of new potential drugs to treat it.
The disease model cells and the genetically matched control mature rapidly, are highly reproducible between batches, and have unprecedented scalability. These key features make the products ideally suited to screening applications for early drug discovery as well as for fundamental research.
The third product announced today is ioGABAergic Neurons. GABAergic neurons are responsible for slowing down the propagation of the brain’s electrical signals. This function is essential for information to be efficiently relayed throughout the brain. GABAergic neuron dysfunction has been associated with a variety of neurological diseases, including epilepsy, schizophrenia, autism and Alzheimer’s disease.
Existing methods used to generate iPSC-derived GABAergic neurons are problematic, as the resulting cell population is heterogeneous with other neuronal cell types, leading to a lack of experimental reproducibility. ioGABAergic Neurons address these challenges allowing scientists to work with an unprecedented pure population of cells (>95% purity) that are highly characterised, functional and are ready for experimentation within days.
Dr Marius Wernig, professor at Stanford and SAB member, said:
“Research into neurological conditions such as FTD are at a pivotal stage. It is exciting to see the progress we are making. And whilst animal models have provided important insights, they differ considerably from human biology. This has contributed to the difficulties translating science into the clinic. The reprogramming approach that my lab has developed for generating GABAergic and glutamatergic neurons, which also forms the basis of bit.bio’s cells and disease models, can help address this gap.”
Dr Farah Patell-Socha, VP Research Products at bit.bio, said:
“The launch of these products is further evidence of our robust CNS cell pipeline for research and drug discovery. Physiologically relevant human cells that perform consistently across lots are game-changing for the industry. Furthermore, this performance alleviates the need for lot-to-lot validation, translating into significant time and cost savings. These products will pave the way for high-throughput screening and drug target validation in human iPSC-derived models that was previously impossible.”
The latest disease models are now available to order or can be pre-ordered.
Scientists can now register their interest and find full physiological data for early access ioGABAergic Neuron vials.
Notes to editors
About bit.bio
bit.bio is a synthetic biology company providing human cells for research, drug discovery and cell therapy. It has released nine ioCells research products into the market and is currently building out its clinical pipeline.
bit.bio’s foundational opti-oxTM precision reprogramming technology enables highly consistent and scalable manufacture of human cells. The activation of cell type-defining transcription factor programs inserted into genomic safe harbour sites allows deterministic reprogramming of induced pluripotent stem cells (iPSC) into highly defined and mature human cells. bit.bio’s discovery platform is based on a deep synthetic biology tech stack and uses large scale experimentation and machine learning to identify combinations of transcription factor genes that encode cell identity.
bit.bio has assembled a team of pioneers with world-leading expertise in stem cell and synthetic biology, manufacturing, and clinical translation. The board is chaired by serial entrepreneur Dr Hermann Hauser and includes Sir Gregory Winter (Nobel Prize for Medicine) and biotech veteran Alan Roemer (co-founder Roivant and Pharmasset).
The company was spun out of the University of Cambridge in 2016. It is at Series B stage and has raised a total of $150M capital from Arch Ventures, Foresite Capital, Milky Way, Charles River Laboratories, National Resilience, Tencent, and Puhua Capital, and others.
For more information visit bit.bio
About ioGABAergic neurons
ioGABAergic Neurons have been precision reprogrammed from human induced pluripotent stem cells (iPSCs) using our precise reprogramming technology: opti-ox (optimized inducible overexpression). Cryopreserved cells, within 12 days post-revival, convert into highly defined (>95%), consistent, mature, and functional GABAergic neurons, providing a high-quality human model for the study of neurological activity and disease.
There is a need for highly defined, functional, and mature models of inhibitory GABAergic neurons to allow academic researchers to investigate the specific contribution of GABAergic neuronsto neurological disease progression, to provide robust platforms for the pharmaceutical industry to test new therapeutics and perform drug screening and, in the future, cell therapy and transplantation. ioGABAergic Neurons derived from human iPSCs using our opti-ox technology opens the avenue to fill the gap in the market and provide researchers, pharma and biotech with a more optimal human cellular model for their studies.
About ioGlutamatergic Neurons MAPT P301S and ioGlutamatergic Neurons MAPT N279K
The ioDisease Model cells take advantage of precision cell reprogramming and CRISPR/Cas9 genetic engineering, to produce highly characterised human induced pluripotent stem cell (hiPSCs)-derived excitatory neurons with disease-relevant mutations..
Frontotemporal dementia (FTD) is the second leading cause of early onset dementia following Alzheimer's disease. It involves atrophy of the frontal and temporal regions of the brain affecting language, memory, and behaviour.
The disease models are engineered with a disease-relevant P301S or N279K mutation in the MAPT gene, which codes for the Tau protein. These mutations in the Tau protein promote hyperphosphorylation and aggregation, which leads to neuronal dysfunction, associated with the pathology of FTD. These disease models offer a fast and easy-to-use system for investigations into the impact of gene function on disease progression. One of the key advantages is the availability of a genetically matched control, which enables researchers to make true comparisons as they can attribute observed differences to the single genetic modification.
View source version on businesswire.com: https://www.businesswire.com/news/home/20221123005007/en/
Contacts
Media Contact
April Six on behalf of bit.bio
bitbio@aprilsix.com
+44 7875 468942













